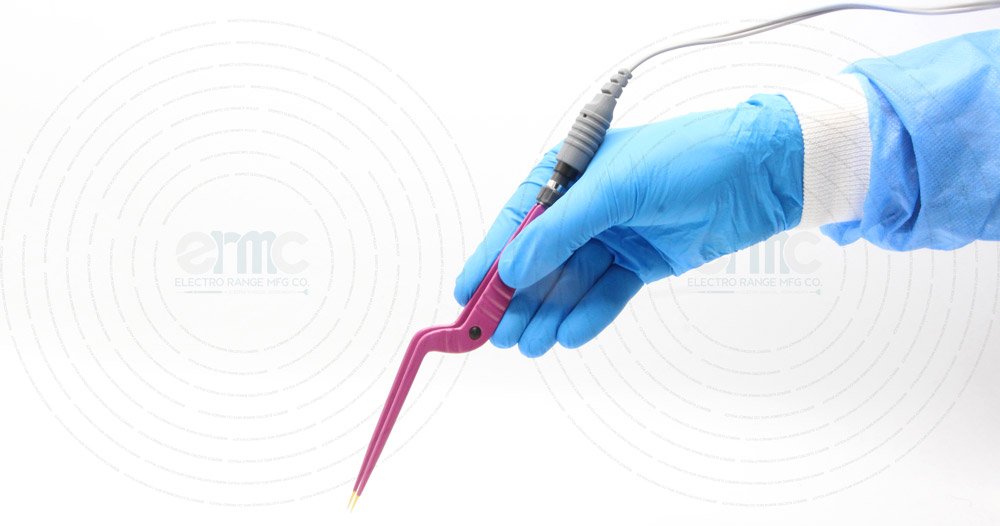
Elegance with Precision
ERMC forceps are engineered with high-quality stainless steel and advanced silver-based alloys, coated with pure epoxy insulation to ensure durability, safety, and accuracy.Designed to deliver non-stick performance, efficient coagulation, and superior handling, they redefine reliability in electrosurgery.
Comfort & Control
The lightweight handle is shaped for comfort and stability, minimizing fatigue while allowing smooth maneuverability during prolonged operations


Autoclave Durability
The connector withstands repeated autoclave sterilization without losing shape or grip. Unlike resin-based connectors that soften and misalign, ERMC connectors are resin-free and molded to international standards, ensuring lasting precision and compatibility with major brands.
Non-Stick Performance
Finely polished gold-plated tips deliver smooth current flow
and reliable coagulation while preventing tissue adhesion.
This results in cleaner procedures, reduced eschar, and
greater surgical precision.


Safety with Balance
A carefully engineered stopper maintains exact tine alignment, ensuring balanced pressure and dependable handling. Surgeons can achieve consistent, repeatable accuracy in every procedure.
Precision Alignment
A carefully engineered stopper maintains exact tine alignment, ensuring balanced pressure and dependable handling. Surgeons can achieve consistent, repeatable accuracy in every procedure.



Is there something else you’re looking for?
WhatsApp us

