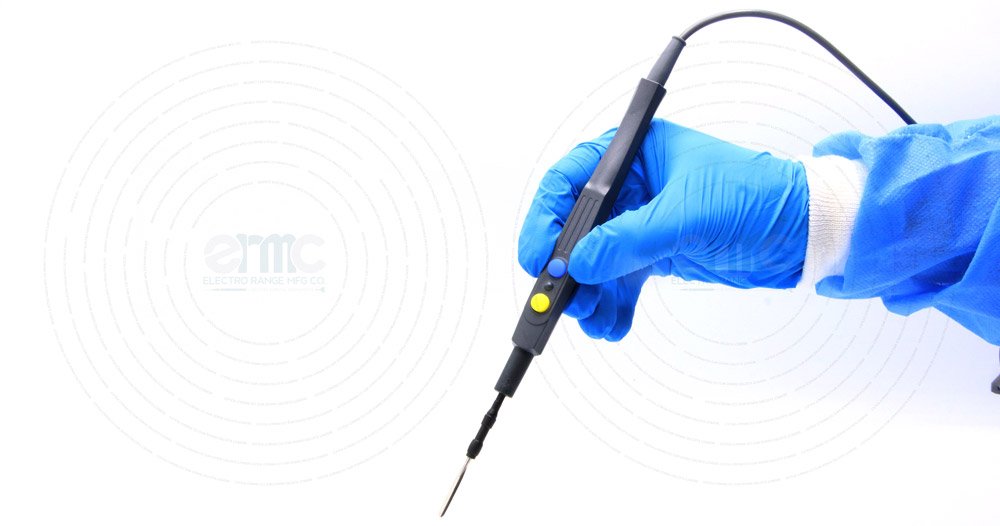
Control at Your Fingertips
ERMC cautery hand pieces are engineered for surgeons who demand precision, comfort, and long-lasting reliability. With a slim ergonomic profile, ultrasonic-sealed body, and proven autoclave resistance, they ensure safe and efficient energy transfer even under the most demanding surgical conditions.
Reliable Safety
Crafted from durable, heat-resistant materials, the hand piece maintains its integrity under extreme conditions. Itsadvanced design allows safe passage of high-voltage current, delivering consistent performance and patientsafety.


Protected Reliability
Each hand piece is manufactured with an ultrasonic-sealed
body that resists high-pressure autoclave steam. This
prevents vapors from compromising the PCB, eliminating the
risk of short circuits and ensuring long-term functionality
after multiple sterilizations.
Precision at a Touch
Equipped with responsive cut and coag buttons, the hand piece delivers precise activation for smooth cutting and coagulation of abdominal tissues and surfaces. Surgeons benefit from enhanced accuracy and confidence in every procedure.

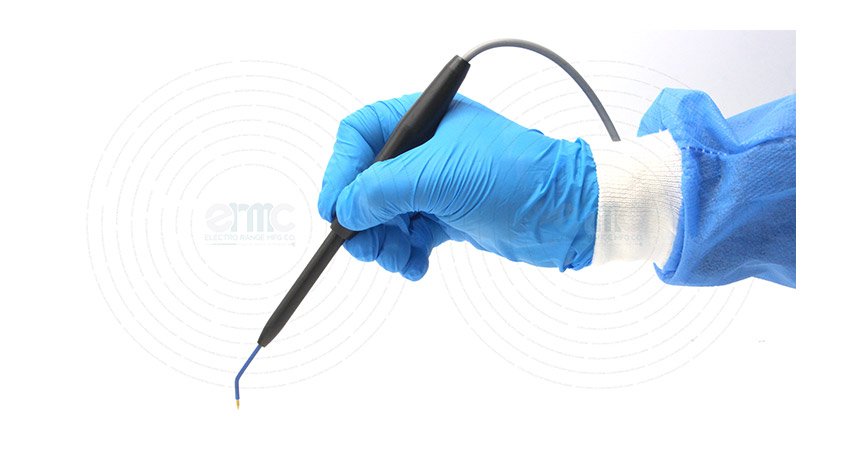
Surgeon Comfort
The lightweight, slim profile minimizes fatigue and enhances maneuverability, giving surgeons superior control during extended operations
Proven Reliability
Built for Multiple surgeries and number of autoclave cycles. ERMC hand piece withstands up to 50 autoclave sterilization cycles, confirming its exceptional durability and long-term value and this standard rarely offered in the market.



Is there something else you’re looking for?
WhatsApp us

